Leading the way in Infection
Prevention and Wound Care Solutions
Bravida is redefining what’s possible in wound care and infection prevention, with a portfolio of transformative products and technologies that are clinically proven to deliver uncompromising freedom and protection.
Why Bravida:
Breakthrough Technology for
Better Healthcare

An antimicrobial cleanser, gel and
irrigation solution that breaks down
and removes micro-organisms in the
wound bed without damaging
healthy cells and tissue

A clear, LATEX FREE, amorphous
hydrogel wound dressing that helps
maintain a moist wound
environment that is conducive
to healing

Anasept® Antimicrobial Skin and Wound Gel is a clear, amorphous, isotonic hydrogel that helps maintain a moist wound environment that is conducive to healing, by either absorbing wound exudate or donating moisture while delivering 0.057% broad-spectrum antimicrobial sodium hypochlorite. Anasept Gel inhibits the growth of bacteria such as Staphlyococcus aureus, Psuedomonas aeruginosa, Escherichia coli, Proteus mirabilis, Serratia marcescens, Acinetobacter baumannii, antibiotic resistant Methicillin Resistant Staphylococcus aureus (MRSA), and Vancomycin resistant Enterococcus faecalis (VRE), Clostridium difficile and Carbapenem Resistant E.coli (CRE) that are commonly found in the wound bed, as well as, fungi such as Candida albicans and Aspergillus niger.
Anasept Antimicrobial Skin and Wound Gel is pure, completely colorless, isotonic, non-cytotoxic, tissue compatible viscous hydrogel. Anasept Antimicrobial Skin & Wound Gel has a 24 month shelf-life when stored at normal room temperature up to 25° C (77° F).
Indications for Use:
Anasept Antimicrobial Gel is intended for OTC use for management of skin abrasions, minor irritations, lacerations, cuts, exit sites and intact skin.
Professional Use:
Anasept® Gel is intended to be used under the supervision of a healthcare professional in the management of wounds such as:
• Partial to full thickness
• Pressure ulcers (Stage I-IV)
• Cuts and abrasions
• Surgical incisions/wounds
• Diabetic foot ulcers
• First and second degree burns
• Venous stasis ulcers
• Grafted wound or donor sites
• Intact and/or irritated skin
Anasept Antimicrobial
Skin and Wound Cleanser



Anasept® is an extremely safe and gentle antimicrobial skin and wound cleanser that contains a broad-spectrum antimicrobial agent sodium hypochlorite. Anasept® is a very pure, completely colorless, isotonic, tissue compatible solution. Anasept® is stable for 24 months at room temperature and is free of necrotizing chemicals such as sodium hydroxide, borates or carbonates found in other antiseptic products. Anasept will not cause those unsightly brown Povidone iodine stains.
Anasept® Antimicrobial Skin and Wound Cleanser is a clear, isotonic liquid that helps in the mechanical removal of the debris and foreign material from the application site. Dirt, debris and foreign materials are mechanically removed by the action of the fluid (Wound Cleanser) moving across the wound bed or application site. Anasept Antimicrobial Skin and Wound Cleanser contains a 0.057% broad-spectrum antimicrobial agent sodium hypochlorite. It inhibits the growth of bacteria such as Staphlyococcus aureus, Psuedomonas aeruginosa, Escherichia coli, Proteus mirabilis, Serratia marcescens, Clostridium difficile, including antibiotic resistant Methicillin Resistant Staphylococcus aureus (MRSA), Vancomycin Resistant Enterococcus faecalis (VRE), Carbapenem Resistant E.coli (CRE) and Acinetobacter baumannii, that are commonly found in wound bed as well as fungi such as Candida albicans and Aspergillus niger.
Professional Use:
Anasept® Antimicrobial Skin and Wound Cleanser is intended for use under the supervision of a healthcare professional for the cleansing of foreign materials including micro-organisms from wounds such as stage I-IV pressure ulcers, diabetic foot ulcers, post surgical wounds, first and second degree burns, grafted and donor sites.
OTC:
Anasept® Antimicrobial Skin & Wound Cleanser is intended for OTC use for mechanical cleansing & removal of dirt, debris & foreign material from skin abrasions, lacerations, minor irritations, cuts, exit sites & intact skin.
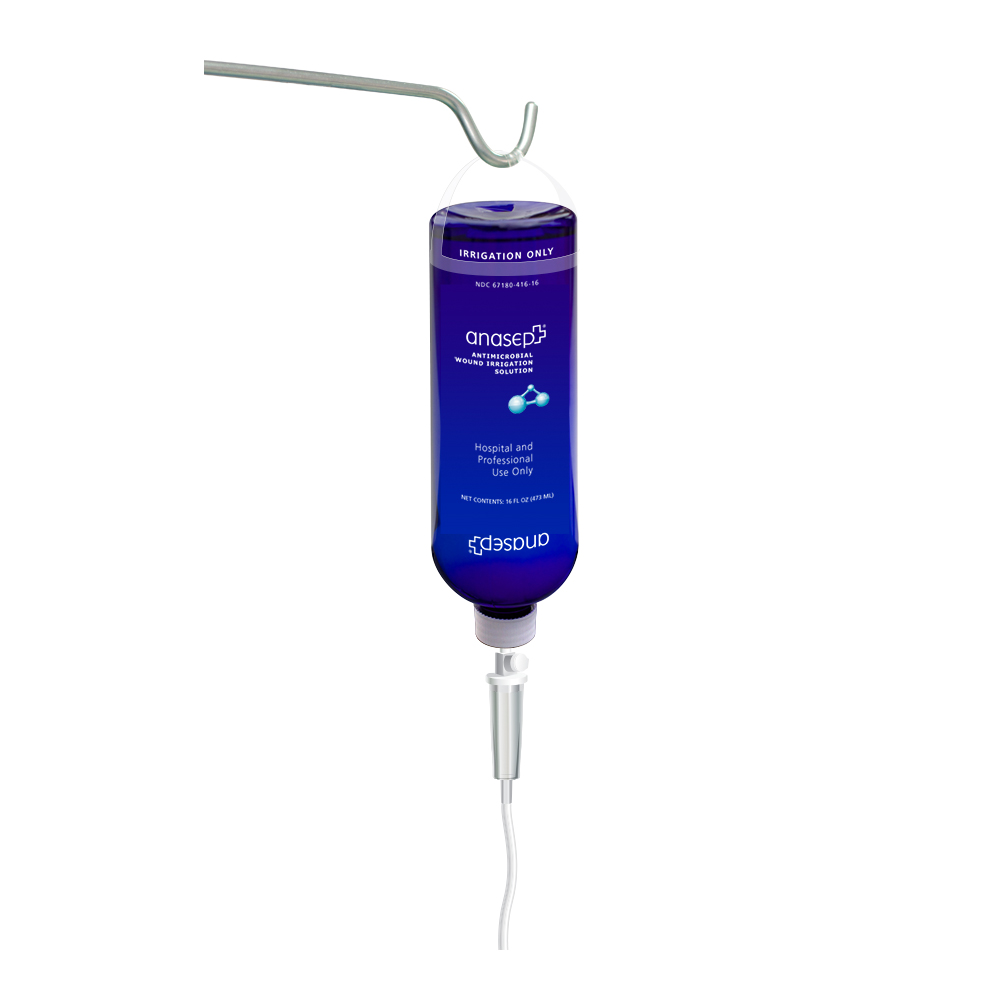
Anasept® Antimicrobial Wound Irrigation Solution is a breakthrough in wound care. Based on Anacapa’s highly regarded Anasept Antimicrobial Skin and Wound Cleanser, this antimicrobial irrigation solution provides a new dimension in antimicrobial wound care and Negative Pressure Wound Therapy.
Anasept® Antimicrobial Wound Irrigation Solution is a clear, isotonic liquid that helps in the mechanical removal of the debris from the application site while delivering 0.057% broad-spectrum antimicrobial sodium hypochlorite via Negative Pressure Wound Therapy Device. Anasept Antimicrobial Wound Irrigation Solution inhibits the growth of bacteria such as: Acinetobacter baumannii, Clostridium difficile, Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Staphylococcus aureus, Serratia marcescens, Carbapenem Resistant Escherichia coli (CRE), Methicillin Resistant Staphylococcus aureus (MRSA), Vancomycin Resistant Enterococcus faecalis (VRE), as well as fungi such as: Candida albicans and Aspergillus niger that are commonly found in the wound bed.
Indications for Use:
Anasept Antimicrobial Wound Irrigation Solution is intended for use under the supervision of a healthcare professional for cleansing of foreign materials including micro-organisms from wounds such as: stage I-IV pressure ulcers, diabetic foot ulcers, post-surgical wounds, first and second degree burns, grafted and donor sites.
Warnings:
For External Use Only. Discontinue use if redness or irritation develops. Do not use in the eyes. Do Not Freeze.

Silver-Sept® Silver Antimicrobial Skin & Wound Gel is a clear, LATEX FREE, amorphous hydrogel wound dressing that helps to maintain a moist wound environment that is conducive to healing. Silver-Sept will absorb a mild amount of wound exudate. Silver-Sept will not stain or discolor tissue.
LONG LASTING:
Silver-Sept functions as a long-lasting, antimicrobial barrier by inhibiting the growth of bacteria including the antibiotic resistant strains: MRSA & VRE, as well as, clinically important fungi such as: Candida albicans and Aspergillus niger.
BIOCOMPATIBILITY AND SAFETY:
Silver-Sept has been subjected to rigorous biocompatibility and safety testing at independent testing laboratories and shown to be non-irritating, non-sensitizing and non-cytotoxic and meets the highest standards for safe use. Testing included:
• Cytotoxicity Studies
• ISO Skin Irritation Studies
• ISO Sensitization Studies
• USP and ISO Modified Systemic Toxicity Studies