- My Account
- Place a Reorder
- Logout
Home » Shop » Hospital Supplies » 3M Health Care 1860 & 1860S N95 Particulate Respirator and Surgical Masks
3M Health Care 1860 & 1860S N95 Particulate Respirator and Surgical Masks
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
DESCRIPTION
DETAILS
Reviews
DESCRIPTION
Solventum (Formerly 3M) Health Care N95 1860 Series Particulate Respirator and Surgical Mask
3M Health Care is now Solventum. The same products you know and trust, now under a new brand.
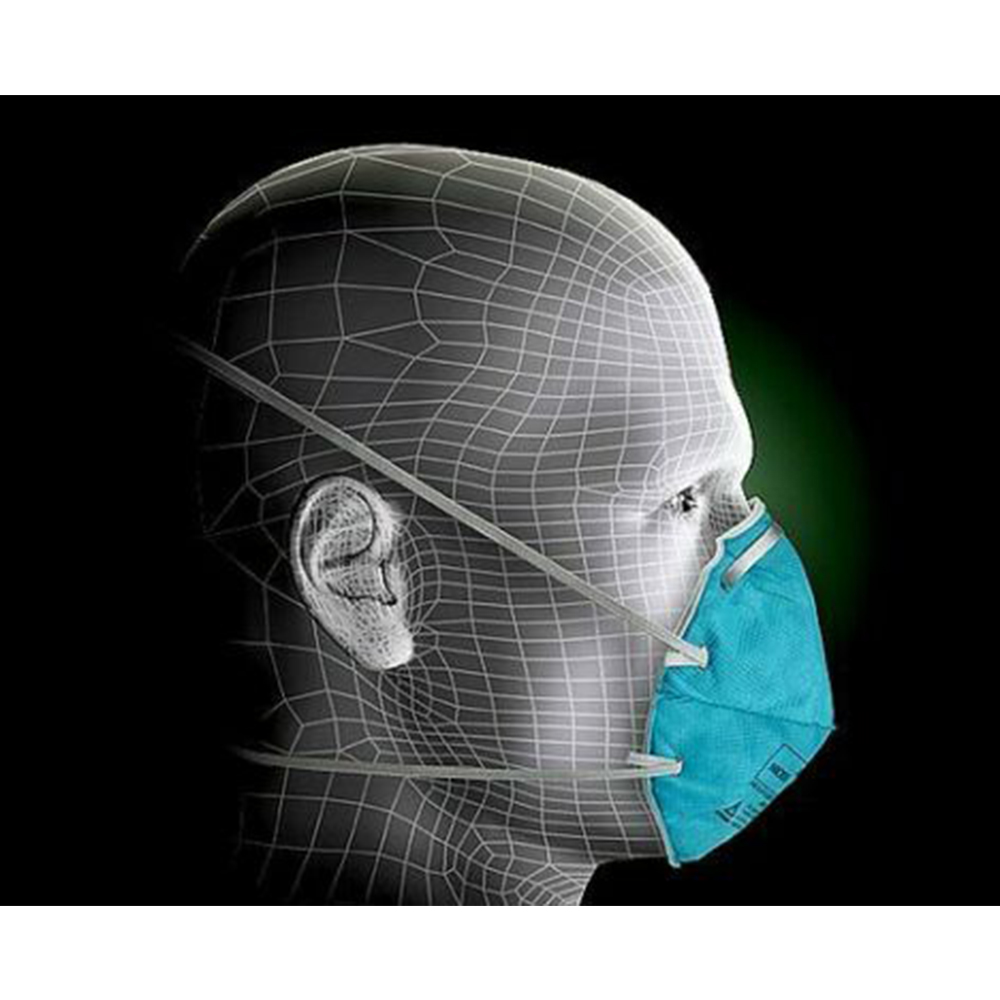
The Solventum (Formerly 3M) Health Care N95 1860 Series Particulate Respirator and Surgical Mask helps provide respiratory protection against certain airborne biological particles. It is disposable and fluid resistant to splash and spatter of blood and other infectious material.
As a disposable particulate respirator, it is intended to help reduce wearer exposure to certain airborne particles, including those generated by electrocautery, laser surgery, and other powered medical instruments. As a surgical mask, it is designed to be fluid-resistant to splash and spatter of blood and other infectious materials. It meets CDC guidelines for Mycobacterium tuberculosis exposure control.

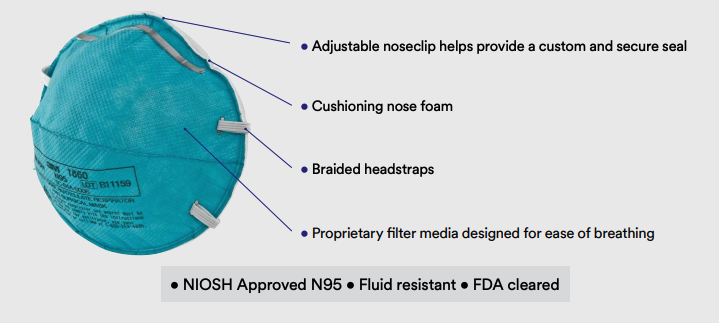
Solventum (Formerly 3M) 1860 Mask Features
- NIOSH approved N95
- Meets CDC guidelines for Mycobacterium tuberculosis exposure control
- FDA cleared for use as a surgical mask
- 99% BFE (Bacterial Filtration Efficiency) according to ASTM F2101
- Fluid resistant according to ASTM F1862
- Respirator contains no components made from natural rubber latex
- Collapse resistant cup shape design
- Braided headbands, cushioning nose foam, and light weight construction for comfortable wear
- Made in the U.S. from globally sourced materials
As a disposable particulate respirator, it is intended to help reduce wearer exposure to certain airborne particles, including those generated by electrocautery, laser surgery, and other powered medical instruments. As a surgical mask, it is designed to be fluid-resistant to splash and spatter of blood and other infectious materials.
This healthcare respirator is designed to help provide respiratory protection for the wearer.

Product Specifications
- Item Number: 1860 & 1860S
- Fluid Resistance: 120 mmHg
- Size: Standard and Small Sizes fit a broad range of faces
- Color: Teal
- Strap Attachment Type: Braided Comfort Strap
- Respirator Style: Cup

How to Use Solventum (Formerly 3M) N95 1860 Series Particulate Respirator and Surgical Masks
- Cup the respirator in your hand with the nosepiece at fingertips, allowing the head straps to hang freely below hand.
- Position the respirator under your chin with the nosepiece up.
- While holding the respirator in place, pull the top strap over your head so it rests high on the back of your head.
- While continuing to hold the respirator firmly in place, pull the bottom strap over your head and position it around your neck, below your ears. Untwist the straps. Position the respirator low on your nose.
- Using both hands, mold the nosepiece to the shape of your nose by pushing inward while moving your fingertips down both sides of the nosepiece.
- To perform the user seal check, place both hands completely over the respirator, being careful not to disturb the position, and exhale sharply. If air leaks around your nose, adjust the nosepiece as described in step 5. If air leaks at respirator edges, adjust the straps back along the sides of your head.
Solventum 1860 Masks FAQs
Is Medical Monks an Authorized Reseller for Solventum 1860 masks?
Yes. Medical Monks and it’s distributor purchase these masks directly from Solventum.
What is the difference between Standard (non-surgical) N95 Respirators and Surgical N95 Respirators?
Standard and surgical N95 respirators are both designed to help reduce the wearer’s exposure to airborne particulate hazards. In addition, surgical N95 respirators are FDA cleared as a medical device and can be used as a fluid barrier to splashes and sprays. Standard and surgical N95 respirators are both NIOSH-approved.
What is the difference in intended use with a Standard (non-surgical) N95 Respirators, Surgical N95 Respirators and Surgical Mask?
N95 Respirators are designed to help reduce the wearer’s exposure to airborne particles.
What can’t I use the Solventum 1860 N95 masks for?
DO NOT use in industrial settings
DO NOT use for gases or vapors (i.e. anesthetic gases such as isoflurane or vaporsfrom sterilants such as glutaraldehyde.)
DO NOT use in any manner not indicated in the User Instructions
What is the respiratory protection for surgical applications?
It is the responsibility of the healthcare organization to determine acceptability of any respirator to help protect their personnel and ensure compliance with the respiratory protection program requirements of the U.S. Federal and/or State OSHA. The healthcare organization must also determine which tasks are considered “surgical procedures,” and if respiratory protection is required for these surgical procedures, they would need to use FDA-cleared surgical N95 respirators for those tasks and a parenthetical (or other configurations approved for emergency use).
Are 3M N95 masks reusable?
N95 filtering face piece respirators can be reused until they are dirty, damaged, or difficult to breathe through. You should inspect your N95 respirator before each time you put it on. If the straps or noseclip are broken, it’s torn, dirty, or otherwise damaged, then you should dispose of it.
When should I change my 3M mask filter?
[Particule Filters should be replaced] when the breathing resistance becomes excessive to the wearer, if any damage occurs, and if it become unhygienic.
Are N95 masks the best for COVID?
Any common face mask provides significant protection against the virus that causes COVID-19, but N95 masks are most effective at slashing the amount emitted by infected people, according to a University of Maryland-led study released Wednesday. So-called “duckbill” N95 masks scored highest in the study, which measured the exhaled breath of participants who were tested both masked and unmasked to measure comparative outputs of SARS-CoV-2. The inexpensive masks, which have two head straps and a horizontal seam, captured 98% of exhaled virus, according to the study published in eBioMedicine.
Downloadable Resources
Product Item Number
1860,1860S
PDF File
DETAILS
Additional information
| Manufacturer | Solventum (Formerly 3M) |
|---|---|
| Mask Type | |
| Product Details | |
| Size | |
| Latex | |
| Features | |
| Amount | Box of 20, Case of 120 |
| Billing Supported | |
| fsa_enabled | true |
Reviews
Most orders ship same day
Straightforward and Streamlined
8am - 7pm EST Monday thru Friday